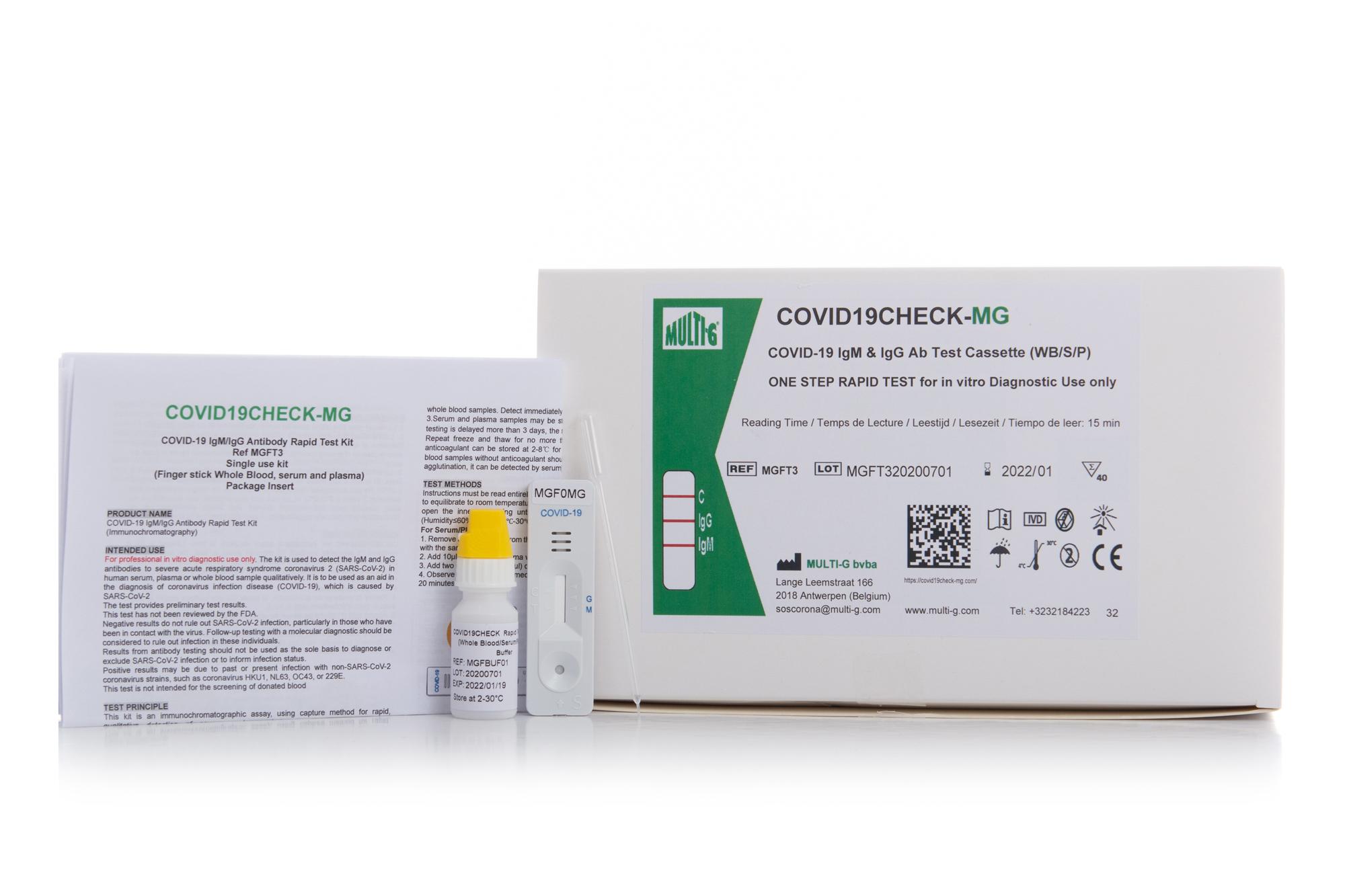
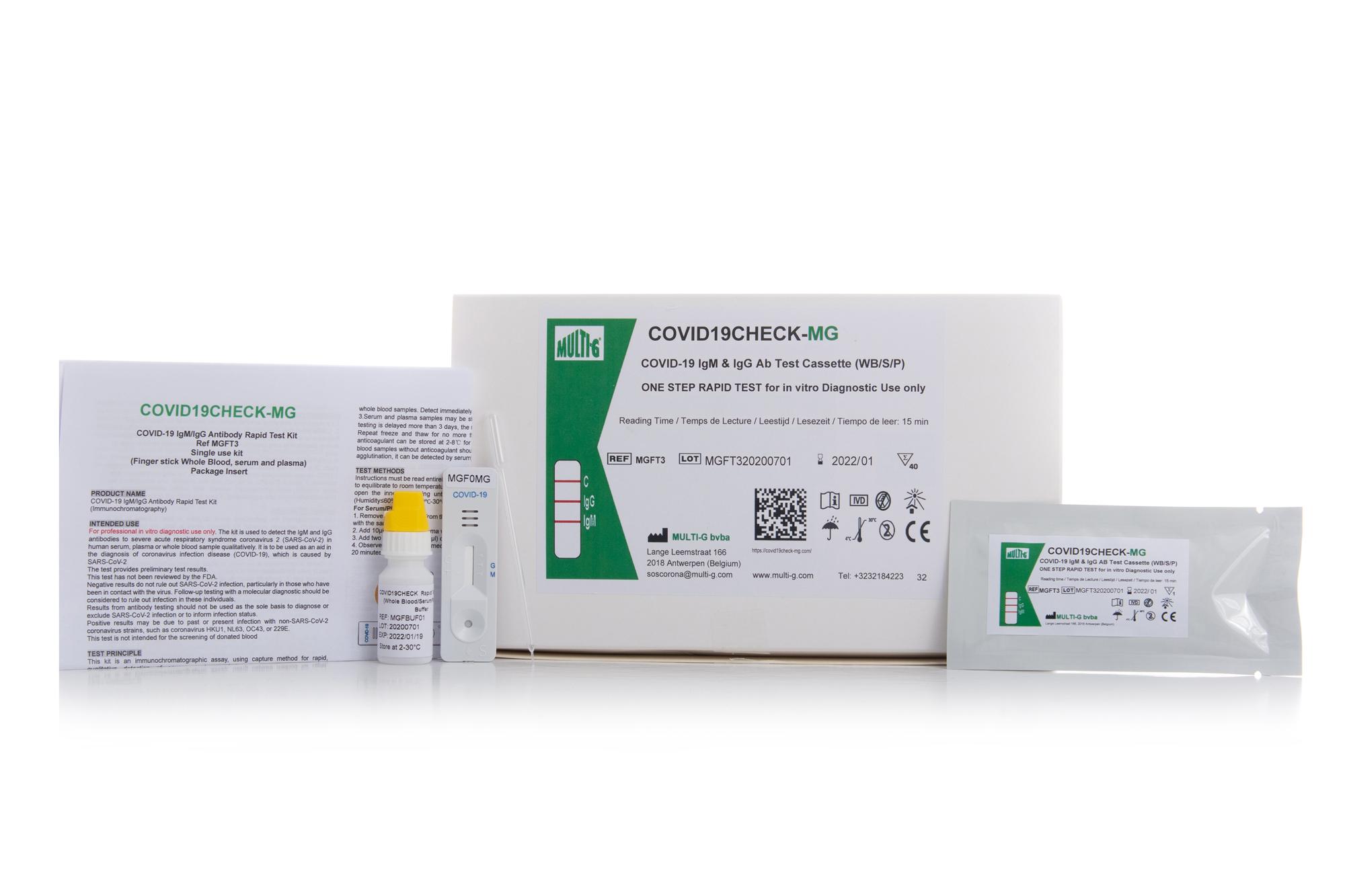
COVID19Check-MG
IgM/IgG Serologic Rapid Test
Multi-G's Covid19Check-MG IgM/IgG Serologic Rapid Test for COVID-19 is used to qualitatively detect IgG and IgM antibodies against SARS-CoV-2 in human serum, plasma or whole blood in vitro.
The test
There is a critical, global need for serology assays that can complement nucleic acid (PCR) tests for diagnosing SARS-CoV-2 infection. Although critically important, PCR tests are only positive during the brief window of acute infection, after which they become negative.
And while serology tests are not as effective as PCR early in acute infection, they are able to detect COVID-19 antibodies for a prolonged period of time after disease resolution, which enables identification of prior infection.
Knowledge of prior infection is epidemiologically important and represents a significant unmet need in the management of the COVID-19 pandemic.
And while serology tests are not as effective as PCR early in acute infection, they are able to detect COVID-19 antibodies for a prolonged period of time after disease resolution, which enables identification of prior infection.
Knowledge of prior infection is epidemiologically important and represents a significant unmet need in the management of the COVID-19 pandemic.
This valuable serological assay could serve as a complementary source of diagnostic information to RT-PCR and chest imaging.
It may also be useful to monitor medical and non-medical workers during the ongoing pandemic or during subsequent waves, and to monitor the immunological status of the general population after social distancing measures have eased.
The assay can be used as a bedside tool, in a laboratory or used in a general practitioner’s office.
This test kit is for healthcare professional use only.
This test kit is for healthcare professional use only.
Features & Benefits
Multi-G's Covid19Check-MG IgM-IgG Serologic Rapid Test for COVID-19 is used to qualitatively detect IgG and IgM antibodies against SARS-CoV-2 in human serum, plasma or whole blood in vitro.
Precise results
- Works with whole blood, serum, & plasma
- Tests for both IgM and IgG antibodies
- Validated using PCR
- Sensitivity : IgG : 92% - IgM : 82%
- Specificity : IgG : 97% - IgM : 94%
- Accuracy (Total coincidence rate):
IgG: 96,5% - IgM: 92,8%
Quick & intuitive feedback
- 10-15 minutes per test
- Intuitive visual interpretation
- No special equipment needed
How it works
Multi-G's Covid19Check-MG is a lateral flow immunoassay used to qualitatively detect IgG and IgM antibodies against SARS-CoV-2 in human serum, plasma or whole blood in vitro.
Video tutorial: https://www.youtube.com/watch?v=MBSzePkLgeE&t=4s
Video tutorial: https://www.youtube.com/watch?v=MBSzePkLgeE&t=4s
1
Collect a blood, serum or plasma sample.
2
Add the sample to the sample well.
3
Place 2 drops of buffer in the sample well.
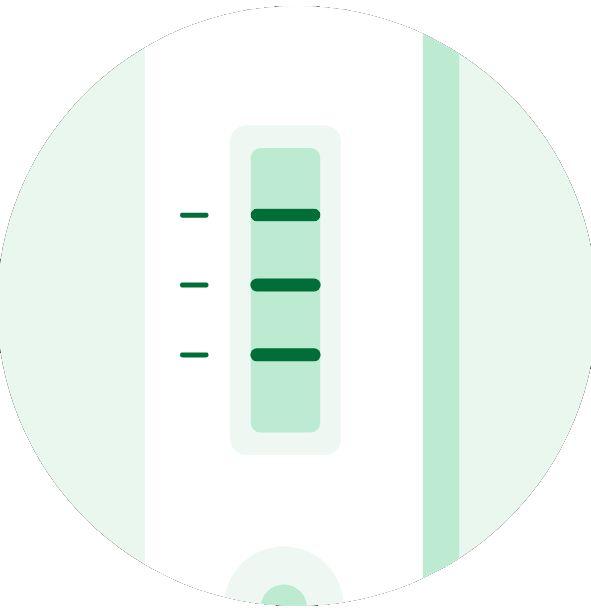
4
Read the results after 10-15 minutes.
Results
A total of three detection lines are possible, with the control (C) line acting as a quality control procedure, appearing only when the sample has flowed through the cassette and the test has worked properly. Clinical significance of antibodies.
Negative
IgG Positive
IgM and IgG positive
Performance
Multi-G's Covid19Check-MG was evaluated at the National Reference Hospital for respiratory pathogens of Leuven in Belgium (KU Leuven). The COVID-19 IgM-IgG Rapid Test is intended to test IgM and IgG separately. The test was validated against a panel of previously samples confirmed with a RT-PCR test. Testing was performed by one operator using one lot of Multi-G's COVID-19 IgM-IgG Rapid Test. In that study, Multi-G's Covid19Check-MG displayed IgG sensitivity of 100% (>12 days after onset of symptoms) and IgG specificity of 97%. The file is available upon request.
Multi-G's Covid19Check-MG have been evaluated and validated by the French Minsitry of Health, and is listed on the French government's official Covid19 website: https://covid-19.sante.gouv.fr/tests
Multi-G's Covid19Check-MG have been evaluated and validated by the French Minsitry of Health, and is listed on the French government's official Covid19 website: https://covid-19.sante.gouv.fr/tests
Quality standards
- Multi-G's Covid19Check-AMG is compliant with CE-IVD regulations.
- Multi-G's production facility is ISO 13485 certified and operates with a pharmaceutical grade clean room.



Applications
Multi-G's Covid19Check-MG covers a range of applications. It is meant for health professionals only. Clinical significance of antibodies.
Epidemiological
applications
Monitor the immunological status of the general population after social distancing measures have eased.
Hospitals, physicians, laboratories & police force
Assess the immune status of first line staff to better manage critical human ressources.
Mining &
oil companies
Assess the immune status of staff in critical environment where social distancing is not possible.
Seroconversion
Assess seroconversion for patients who have been vaccinated against COVID-19.
Herd immunity
Assess herd immunity to better manage COVID-19 restrictions.