COVID19Check-SAL
Antigenic Rapid Test - Saliva sample
It is non invasive and painless. It allows easy and hassle free multiple testing. Covid19Check-SAL detects the known variants of the Sars-Cov-2 virus.
The test
There is a critical, global need for rapid antigen test that can complement nucleic acid (PCR) tests for diagnosing SARS-CoV-2 infection. Although critically important, PCR tests are not always available and can last too long in obtaining the result due to the large amount of people who need testing.
Additionally there is growing concern about the acceptability of the very uncomfortable nasopharyngeal swab sampling, especially as testing it is becoming more frequent to secure most daily activities.
Covid19Check-SAL is an efficient and reliable solution to detect infection in cases where the viral load is significant (Ct <30) and the person is likely to be contagious.
Covid19Check-SAL is not only painless and hassle free, but also has a sensitivity of 97.6% for Ct values up to 36 (the CDC suggests that when the Ct value is >33, the subject is not contagious anymore)
It is particularly useful in cases where frequent testing is required thanks to is simple, clean and pain free saliva sampling method.
Features & Benefits
Precise results
- Sensitivity / PPA : 97.6%
- Sensitivity day 1-5: 96.3%
- Specificity / NPA : 99.1%
- Total coincidence rate : 98.7%
Quick & intuitive feedback
- 15 minutes per test
- Painless
- Clean (no spitting, ni pipetting)
- No training needed (vs nasopharyngeal)
- Intuitive visual interpretation
- No special equipment needed
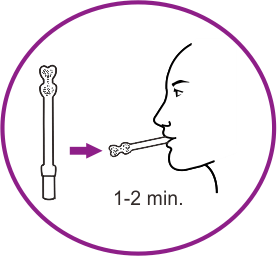
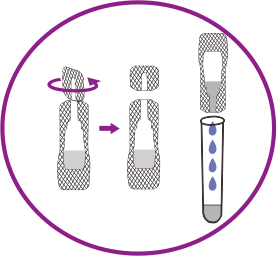
How it works
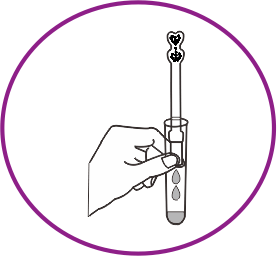

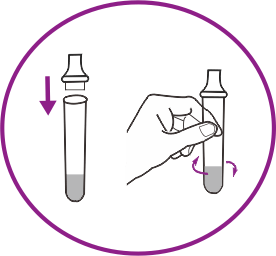
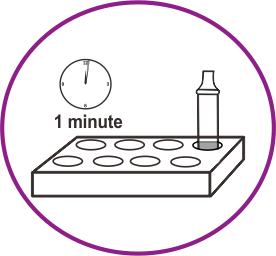
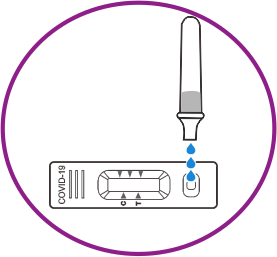
Results
Negative
Positive
Performance
Covid19Check-SAL is a very robust device, it shows no cross reaction with a large list of pathogens (H1N1 (new type A H1N1 influenza virus (2009), seasonal H1N1 influenza virus), H3N2, H5N1, H7N9, influenza B Yamagata, Victoria, respiratory syncytial virus, rhinovirus groups A, B, and C, adenovirus 1, Types 2, 3, 4, 5, 7, 55, enterovirus groups A, B, C, D, Epstein‐Barr virus, measles virus, human cytomegalovirus, rotavirus, norovirus, mumps virus, varicella‐band Herpes virus. No interference were found with 15 substances such as Mucin, Hemoglobin, Histamine Hydrochloride , Human albumin , α‐ interferon , Lopinavir , Tobramycin, Ribavirin , Tramadol, Azithromycin, Meropenem, Oseltamivir, Benzocaine, Peramivir.
Quality standards
In order to ensure the highest level of quality and reliability, Multi-G manufactures its test devices in Belgium, unlike most competitors who simply rebrand Asian made tests.
- Multi-G's Covid19Check-SAL is compliant with the current CE-IVD regulations.
- Multi-G's production facility in Antwerp, Belgium, is LQRA ISO 13485:2016 certified.
- The production facility operates at the highest pharmaceutical standards.
- Covid19Check-SAL is validated by the Belgian Health Agency (https://www.afmps.be/sites/default/files/content/Belgian%20validation.xlsx)



Applications
It is suited for a variety of settings such as companies, pharmacies, airports, schools, universities, sports premises etc.
It is meant for health professionals only.