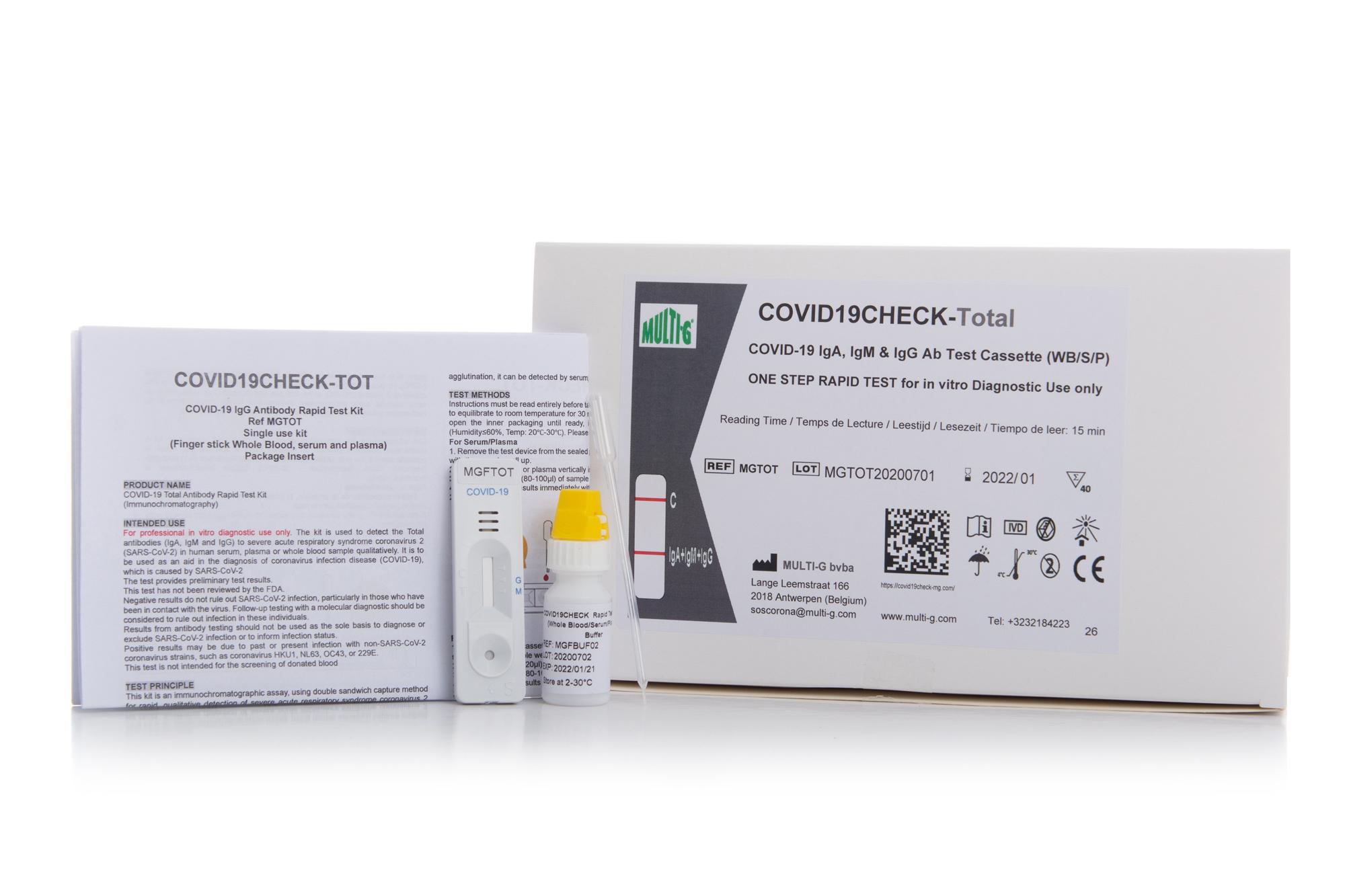
COVID19Check-TOT
IgA+IgM+IgG Serologic Rapid Test
Multi-G's Covid19Check-TOT IgA+IgM+IgG Serologic Rapid Test for COVID-19 is used to qualitatively detect IgA, IgM and IgG antibodies against SARS-CoV-2 in human serum, plasma or whole blood in vitro.
The test
There is a critical, global need for serology assays that can complement nucleic acid (PCR) tests for diagnosing SARS-CoV-2 infection and following up on the patients reaction to the virus. Although critically important, PCR tests are only positive during the brief window of acute infection, after which they become negative.
And while serology tests are not as effective as PCR early in acute infection, they are able to detect COVID-19 antibodies for a prolonged period of time after disease resolution, which enables identification of prior infection.
Knowledge of prior infection is important for epidemiological analysis and represents a significant unmet need in the management of the COVID-19 pandemic as well as a critical clinical information for the physician.
And while serology tests are not as effective as PCR early in acute infection, they are able to detect COVID-19 antibodies for a prolonged period of time after disease resolution, which enables identification of prior infection.
Knowledge of prior infection is important for epidemiological analysis and represents a significant unmet need in the management of the COVID-19 pandemic as well as a critical clinical information for the physician.
This valuable serological assay provides the physician with key data about the patient's immmune response to the virus even from the very early stages, thanks to the detection of IgA, which is the first reaction of the immune system.
It may also be useful to monitor medical and non-medical workers during the ongoing pandemic or during subsequent waves, and to monitor the immunological status of the general population after social distancing measures have eased and better design social distances policies.
The assay can be used as a bedside tool, in a laboratory or used in a general practitioner’s office.
This test kit is for healthcare professional use only.
This test kit is for healthcare professional use only.
Features & Benefits
Multi-G's Covid19Check-TOT IgA+IgM+IgG Serologic Rapid Test for COVID-19 is used to qualitatively detect SARS-CoV-2 total IgA, IgM and IgG antibodies in human serum, plasma or whole blood in vitro. Detecting total antibodies on one single line provides earlier detection and higher performance, therefore allowing to detect all cases of seroconversion.
Precise results
- Sensitivity / PPA: IgA+IgM+IgG : 100% (after 15 days) - 96.25% (global)
- Specificity /NPA : Iga+IgM+IgG : 95.65%
Quick & intuitive feedback
- 10-15 minutes per test
- Intuitive visual interpretation
- One single line for all 3 antibodies
- No special equipment needed
- Portable reader device available on demand
How it works
Multi-G's Covid19Check-TOT is a lateral flow immunoassay used to qualitatively detect total IgA+IgM+IgG antibodies against SARS-CoV-2 in human serum, plasma or whole blood in vitro.
1
Collect a blood, serum or plasma sample.
2
Add the sample to the sample well.
3
Place 2 drops of buffer in the sample well.
4
Read the results after 10-15 minutes.
Results
A total of two detection lines are possible, with the control (C) line acting as a quality control procedure, appearing only when the sample has flowed through the cassette and the test has worked properly Clinical significance of antibodies.
Negative
IgA+IgM+IgG Positive
Performance
Multi-G's Covid19Check-TOT was evaluated in a multi-center study in Belgium, leaded by the Oost-Limburg Hospital, and including several hospitals including GZA (Antwerp) and UZG (Ghent University Hospital). In addition to its regional function, the Oost-Limburg Hospital fulfills a number of important expert functions within a Euroregional context (MIC, NIC, cardiac surgery, neurosurgery, lithotripsy, NMR, fertility center, radiotherapy,…). The test was validated against a panel of samples previously confirmed with a RT-PCR test. Testing was performed by one operator using one lot of Multi-G's COVID-19 TOT Rapid Test. In that study, Multi-G's Covid19Check-TOT displayed total IgA+IgM+IgG sensitivity as high as 100%, and specificity of 96.25%.
Quality standards
- In order to ensure the highest level of quality and reliability, Multi-G manufactures its test devices in Belgium, unlike most competitors who simple rebrand asian made tests.
- Multi-G's Covid19Check-TOT is compliant with CE-IVD regulations.
- Multi-G's production facility in Antwerp, Belgium, is LQRA ISO 13485:2016 approved.
- The production facility operates at the highest pharmaceutical standards.



Applications
Multi-G's Covid19Check-TOT covers a range of applications. It is meant for health professionals only. Clinical significance of antibodies.
Epidemiological
applications
Monitor the immunological status of the general population after social distancing measures have eased.
Hospitals, physicians, laboratories & police force
Monitor the immune status of critical front line staff to better manage human ressources.
Mining &
oil companies
Monitor the immune status of staff in critical settings such as mines and rigs where social distancing is difficult to enforce to ensure staff safety and avoid infection outburst.
Seroconversion
Assess seroconversion and immune response for patients who have been vaccinated COVID-19.
Herd immunity
Assess herd immunity to better manage COVID-19 restrictions policies.